Metabolic plasticity and regulation of mitochondrial bioenergetics in cancer cells
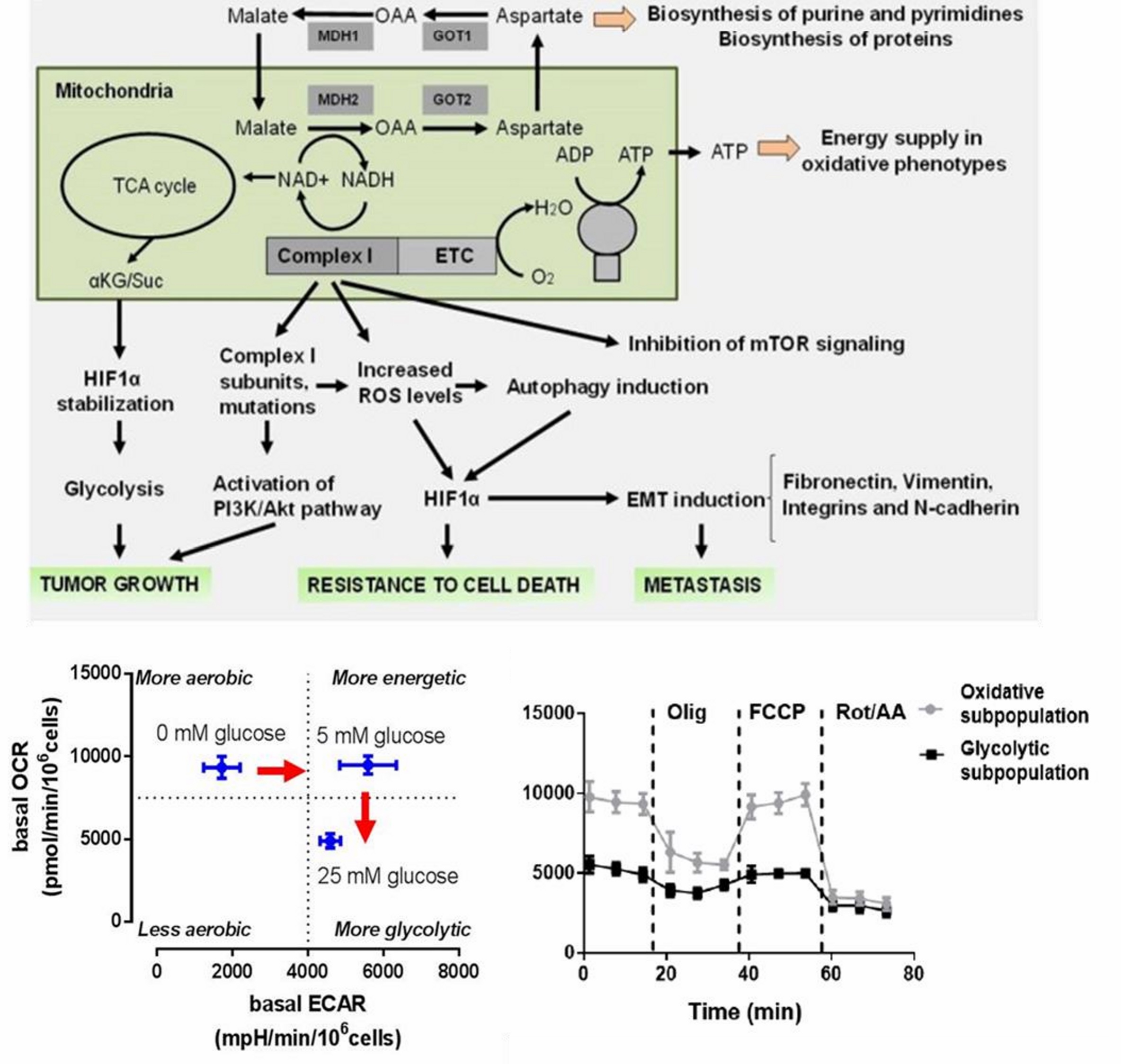
Under metabolic stress stimuli, energy or biosynthetic demands are supplied through dynamic changes in the metabolism. This process, known as metabolic plasticity, allows certain cell subpopulations to remodel the biochemical pathways related with energy production (e.g. metabolic shifts between glycolysis versus oxidative phosphorylation), preference by mitochondrial oxidable substrates (e.g. pyruvate, glutamine oxidation versus fatty acid oxidation) and synthesis of intermediates of tricarboxylic acid (TCA) cycle (e.g. induction of reductive carboxylation versus oxidative decarboxylation), which depend on changes of substrate availability, such as oxygen, glucose, and amino acids.
Currently, metabolic adaptations based on mitochondrial bioenergetics in several physiological processes (e.g. immunological response, platelet activation, survival of stem cells) are understanding; however, the mechanisms of metabolic adaptations that support the tumorigenesis and their genetic interplay remains less well known.
In the MPB lab, we explore the dynamic changes in the metabolic signaling during metastatic cascade and resistance to oncological drugs using real-time approaches based on fluorescent microscopy and metabolic changes analysis. This unexplored topic in cancer biology offers the opportunity to identify novel therapeutic targets for cancer treatment.
Identification of new compounds that target the mitochondrial bioenergetics in cancer cells and platelets
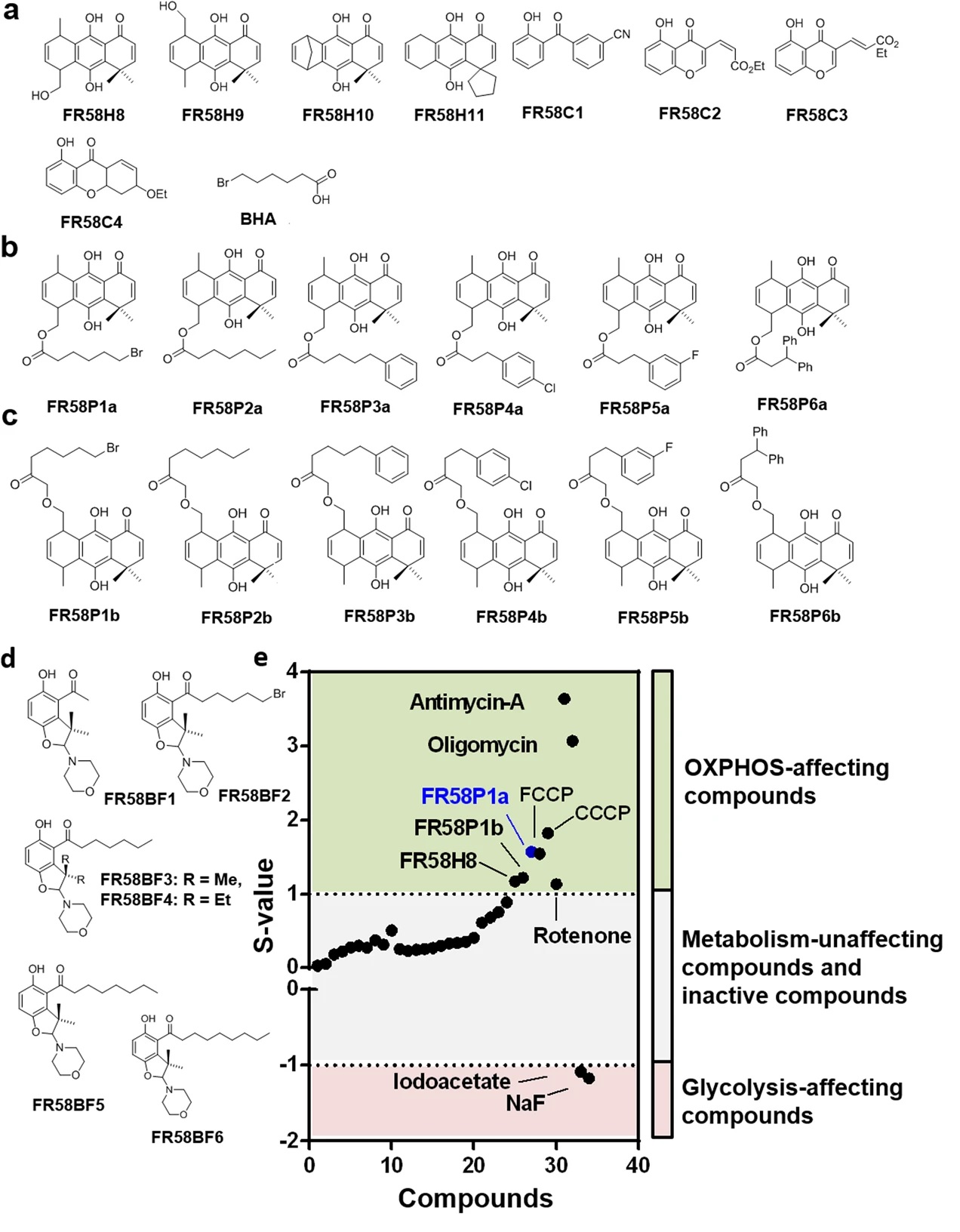
The current therapeutic efficacy of cytostatic drugs is defined by the ability to produce tumor shrinkage; however, this parameter is not predictive of an anti-metastatic effect. Moreover, although the metastasis produces more than 90% of mortality in patients treated with chemotherapy, selective anti-invasion and anti-metastatic (migrastatics) drugs are missing. Recently, new migrastatic small molecules interfering with the actin stability, contractility and ROCK/MLC kinases-dependent migratory signaling have been described in-depth, but the inhibition of migratory/invasive abilities by disruption of mitochondrial metabolism-dependent migratory signaling in cancer cells by OXPHOS-affecting small molecules remain poorly explored.
On the other hand, mitochondrial bioenergetics is essential during the platelet activation-related diseases, whose pharmacological intervention may reduce the thrombosis risk. In the MPB lab, we screen new small molecules and toxins obtained from organic synthesis and natural sources (e.g. snake venoms and amphibian poison secretions), respectively, that target the mitochondrial bioenergetics. Some small molecules described by us are inhibitors of respiratory complex I and α-ketoglutarate dehydrogenase or protonophoric uncouplers of OXPHOS.
Currently, MPB Lab explores 1) the effect of mitochondria-targeted small molecules using lipophilic cations as organelle-specific drug delivery systems on the proliferation of stem cancer cells and migration/invasion of breast cancer cells, 2) the effect of new toxins on mitochondrial morphology and bioenergetics of cancer cells, and 3) new small molecules with inhibitory effects on platelet aggregation by interfering mitochondrial bioenergetics.
Clinical and molecular implications of snakebites in Chile
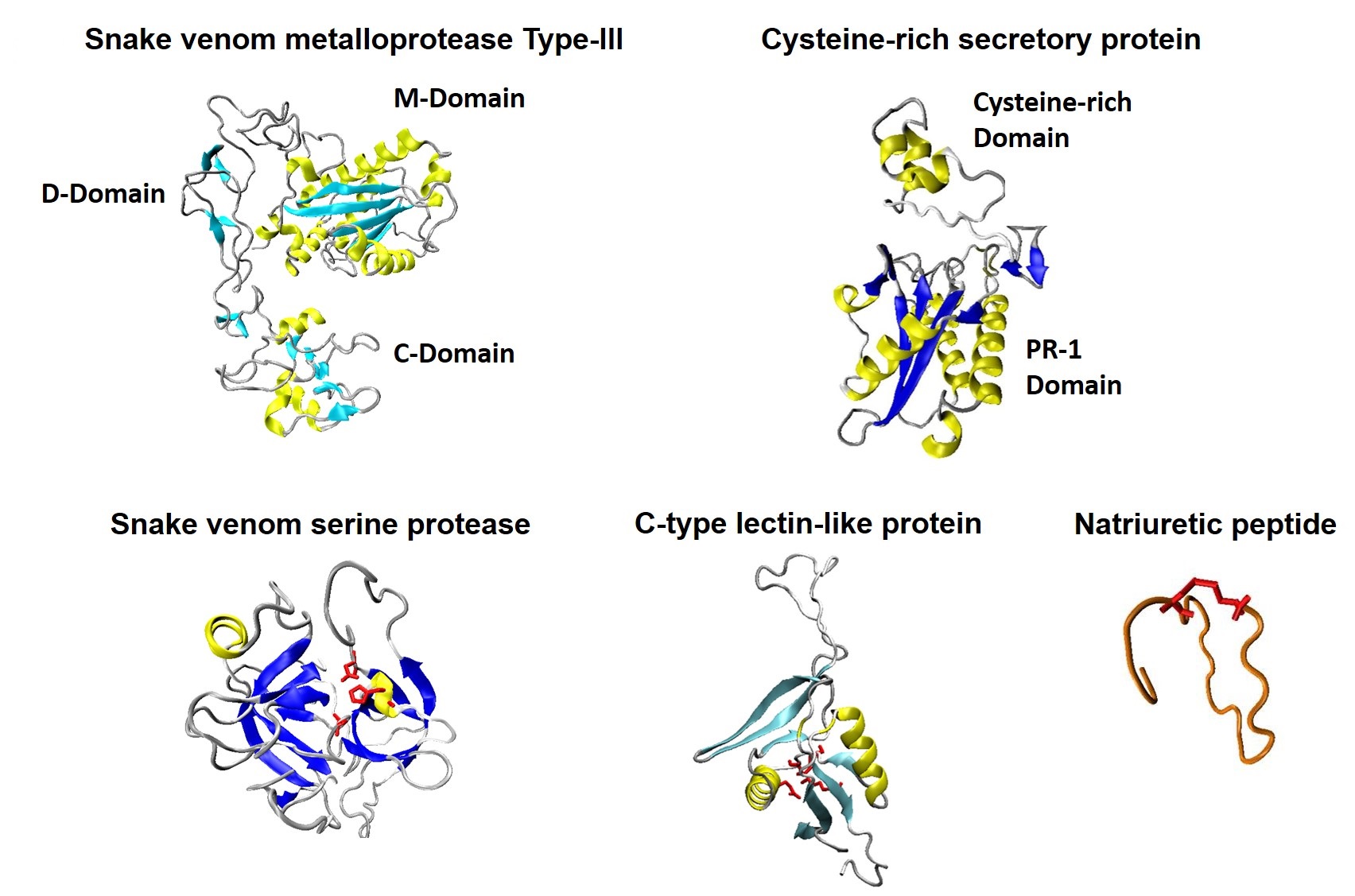
In parallel, in the MPB lab are interested in the translating our studies about drug discovery based on toxins-encoding genes in the clinical and molecular implications of snakebites in Chile. The ophidian accident was recently recognized as a new Neglected Disease by World Health Organization (WHO). Analyzing the historical literature from 1835 on snakes in Chile, we have demonstrated that the knowledge about the clinical and pharmacological management of snakebites is scarce and based on empiric decisions, lacking information about venom compositions and biochemistry characterizations of the toxins of Philodryas and Tachymenis species. Our Laboratory identified for the first time some toxin-encoding genes for a Chilean species, revealing the complex evolutionary relationships with snakes with mortal venom of Elapidae and Viperidae families. Currently, we study/identify 1) the toxins-encoding genes from rear-fanged snakes Philodryas and Tachymenis and their clinical implications; 2) structural variations of venom delivery system and their evolutionary relationships with South American snakes.
